CDC expects monkeypox cases will continue to climb through August
[ad_1]
Federal health officials are warning that they now expect that the number of monkeypox cases will continue to swell through at least August, as the Biden administration says it is changing the way it will ration out vaccines to combat the growing outbreak.
As of Friday, at least 1,814 cases have been tallied by the Centers for Disease Control and Prevention. New York makes up the largest share of cases, with 489 tallied by the agency in the state.
“Now as we closely monitor cases, I would like you to all understand that we anticipate an increase in cases in the coming weeks,” CDC Director Dr. Rochelle Walensky told reporters on Friday.
Some of the looming uptick will simply be the result of better testing for the disease and streamlined paperwork for states to report new cases to the Biden administration, Walensky said.
Smith Collection/Gado/Getty Images
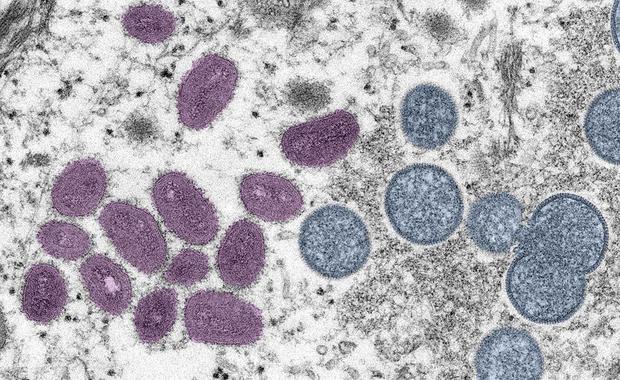
However, given the sheer number of cases of the virus, which can lead to weeks of painful rashes and scarring, health authorities expect many early infections have not yet been detected.
“We know monkeypox’s symptoms usually start within three weeks of exposure to the virus. So we anticipate we may see an increase in cases throughout the month of July and then to August,” Walensky said.
What’s known about cases so far
Walensky said that the CDC’s insight into the details of the outbreak, which now spans at least 43 states as well as the District of Columbia and Puerto Rico, remains limited.
While monkeypox is a “reportable” disease for health departments, the agency cannot compel state and local health authorities to collect and report additional demographic details around each case.
CDC only has demographic details like gender and age for less than half of the cases to date. The “vast majority” of those known cases were in men who have sex with men, though a small handful of women have also tested positive.
The average age in the outbreak, among known cases, is 36 years old. Patients range in age from 18 years old at the youngest to 76 at the oldest.
“We are working with our state and jurisdictional partners to work through data use agreements so that we have that information, but right now, it is voluntarily reported,” Walensky said.
Ramping up vaccinations
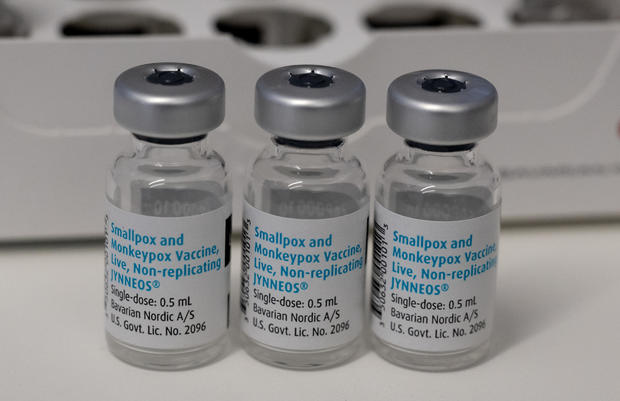
Walensky said the Biden administration was moving to a new phase of its strategy to allocate doses of vaccine around the country, acknowledging that demand still far outstrips available supply for Bavarian Nordic’s two dose Jynneos vaccine.
“The updated strategy will now prioritize vaccine allocation to areas with increasing case burden, while still providing vaccine to all jurisdictions with individuals at increased risk for disease,” said Walensky.
That new formula will govern the distribution of 131,000 more doses of Jynneos vaccine that are now being made available to health departments after being delivered to the country’s stockpile this week.
The new strategy comes as the Biden administration has faced heavy criticism from local officials in communities now facing a spike in monkeypox infections, who have been demanding more vaccines.
Federal health officials rebutted accusations that the Food and Drug Administration had dragged its feet on an inspection of a new production line spun up by Bavarian Nordic to produce some 786,000 doses of vaccine still awaiting release by U.S. regulators.
Sven Hoppe/picture alliance via Getty Images
“Contrary to missing a chance for approval, FDA actively reached out using its contacts with the Biomedical Advanced Research and Development Authority to actually move up the submission that was necessary, and all of the other events that were necessary, to get those doses to be able to used,” the FDA’s Dr. Peter Marks told reporters.
Marks acknowledged that the agency’s European counterparts had already signed off on the facility, but said the FDA did not have an agreement for “mutual recognition of vaccines inspections for initial licensures in from other countries.”
A spokesperson for Bavarian Nordic said in a statement that the company had initially planned to submit paperwork by August for an inspection of the new part of its facility scheduled in the fall, but accelerated the process as the outbreak grew.
Previous doses shipped to the U.S. had been filled and finished into vials by a contractor, the company said.
An FDA spokesperson said the regulator had completed the physical part of its inspection of the new production area with no major red flags, allowing health officials to “pre-position” doses in the U.S. pending the final sign-off.
Combined with an additional U.S. order announced Friday of 2.5 million doses of vaccine to be filled into vials from U.S. stocks, the Biden administration says it hopes to have “nearly 7 million doses by mid-2023.”
“While the supply of Jynneos vaccine has been limited in recent weeks, we are starting to see supply increase, and as it does, we are going to continue getting those doses out as quickly, efficiently and equitably as possible,” Dawn O’Connell, the Assistant Secretary for Preparedness and Response, told reporters.
[ad_2]
Source link